Welcome to a breakfast seminar with Pau Bernadó!
When: Wednesday May 31,09:00 - 10:30
Where: at LINXS (Scheelvägen 19, Lund), with digital participation possibility (Zoom). Registered participants (online-only) will receive a zoom-link the same day as the event.
Title: A Structural Perspective of the Pathological Threshold in Huntington’s Disease
Speaker: Pau Bernadó, Centre de Biologie Structurale (CBS). Montpellier (France).
Abstract:
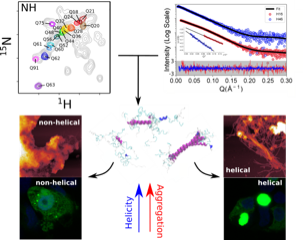
Huntington’s Disease (HD) is a deadly neurodegenerative disorder caused by an expansion of the CAG codons in the first exon of the HTT gene that, after translation, results in an extended poly-glutamine (poly-Q) tract in the N-terminal region of the protein huntingtin (httex1). Only individuals with more than 35 consecutive glutamines in this track develop HD. The structural changes occurring to httex1 when increasing its length beyond this pathological threshold remain poorly understood, precluding a molecular understanding of the pathology. The high-resolution structural investigation of httex1 had been considered as impossible due to its intrinsic flexibility, which preclude the use of X-ray crystallography and cryo-Electron microscopy, and the strong compositional bias that hampers the use of traditional NMR approaches. During the last years, our group has developed chemical biology approaches to incorporate isotopically labelled amino acids into proteins in a site-specific manner, enabling the high-resolution NMR investigation of highly repetitive proteins, such as httex1.
The systematic application of site-specific isotopic labelling has enabled the residue-specific NMR structural characterization of poly-Q tracts for sub-pathogenic (Q16) and pathogenic (Q36, Q46 and Q66) versions of httex1. These NMR data have been integrated with SAXS, SANS and computational approaches, to derive ensemble models and define the rules governing the structure of the poly-Q. Our analysis reveals that the poly-Q tract adopts long α-helical conformations propagated and stabilized by glutamine side chain to backbone hydrogen bonds, and highlights the relevance of the flanking regions in defining this structure. From a biomedical perspective, we show that α-helical stability is a stronger signature than the number of glutamines in defining aggregation kinetics, both in vitro and in cells, and the structure of the resulting fibrils. Our observations provide a structural perspective of the pathogenicity of expanded httex1 and pave the way to a deeper understanding of poly-Q-related diseases.
Organisers: For specific questions about the event, please contact Marie Skepö marie.skepo@teokem.lu.se
Contact: Please contact either nina.ahlbeck@fsi..lu.se or anna.ntinidou@linxs.lu.se for any practical questions.
During our events we sometimes take photographs and short film clips to profile our activities. Please let us know if you don’t want to be in any photos/films before we start the event. Some webinars are recorded to be used for educational purposes in the LINXS website.
By registering to our events you give your permission to LINXS, according to the General Data Protection Regulation (GDPR), to register your name and e-mail address to be used for the sole purpose of distributing newsletters and communications on LINXS activities.